Difference between revisions of "Workshop on Clinical Trial Ontology"
m |
|||
| (274 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
| − | == General Information == | + | == '''General Information''' == |
| − | The National Center for Biomedical Ontology will host a two-day workshop focused on the Ontology of Clinical Trials. The workshop will take place in | + | The National Center for Biomedical Ontology will host a two-day workshop focused on the Ontology of Clinical Trials. The workshop will take place on May 16-17, 2007 at the NIH Campus in Bethesda, MD. |
| − | + | The meeting will be divided into two parts. Day 1 will be a public event consisting of presentations and panel discussions on the CTO and cognate initiatives. Day 2 will be a working meeting devoted to intense discussions of mature drafts of the CTO and to the creation of a strategy for its further development and testing. | |
| − | The | + | The work of the NCBO is funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant 1 U 54 HG004028. Information on the National Centers for Biomedical Computing can be found [http://nihroadmap.nih.gov/bioinformatics here]. |
| − | + | We are grateful to the National Heart, Lung and Blood Institute for generous support for this meeting. | |
| − | + | == '''Aim of the Workshop''' == | |
| + | |||
| + | The aim of the workshop is to foster the creation and dissemination of a reference ontology (high-quality controlled structured vocabulary) for the annotation of the results of clinical trials. | ||
| + | |||
| + | The Workshop will bring together representatives of all of the major groups involved in clinical trial informatics, design, execution, analysis and standardization, with the goal of achieving a broad consensus on the requirements which the ontology should address and on the most effective means of realizing these requirements. It will address the potential uses of CTO, and methodology for CTO design and development. | ||
| + | |||
| + | == '''Goals of the Clinical Trial Ontology Initiative''' == | ||
| + | |||
| + | The proposed CTO should: | ||
(1) fully and faithfully capture the types of entities and relationships involved in clinical trials of any experimental design | (1) fully and faithfully capture the types of entities and relationships involved in clinical trials of any experimental design | ||
| − | |||
| − | |||
| − | |||
| − | + | (2) comprehend terms like: ''cohort, randomization, placebo, response, efficacy, control, protocol, null hypothesis, confidence interval, finding, biomarker, primary outcome, secondary outcome, intervention group, confounder,'' etc., including also major relevant statistical terms and terms drawn from resources such as the CDISC [http://www.cdisc.org/glossary/clinicalterminologyv4.pdf glossary] and | |
| + | all terms needed for the task of meta-analysis of clinical trials; | ||
| + | |||
| + | (3) organize these terms in a structured way, providing definitions and logical relations designed to enhance retrieval of, reasoning with, and integration of the data annotated in its terms. | ||
| + | |||
| + | (4) support trial bank interoperation | ||
| + | |||
| + | (5) form an integral part of the more comprehensive framework of the Ontology for Biomedical Investigations [http://obi.sourceforge.net/ OBI], which itself forms part of the [http://obofoundry.org/ OBO Foundry] | ||
| + | |||
| + | (6) draw on and seek maximal alignment with existing clinical trial ontologies, including: | ||
| + | |||
| + | -- the [http://smi.stanford.edu/smi-web/research/details.jsp?PubId=1227 Epoch ontology] used by the [http://www.immunetolerance.org Immune Tolerance Network] | ||
| + | |||
| + | -- the [http://rctbank.ucsf.edu/ontology/outline/index.htm RCT Schema] ontology used by the[http://rctbank.ucsf.edu/ Trial Bank Initiative] | ||
| + | |||
| + | (7) rest on a clear understanding of the relation between CTO and related ventures such as an epidemiology study ontology | ||
| + | |||
| + | (8) rest on a clear understanding of the relation between CTO and data-model-oriented initiatives such as CDISC and BRIDG | ||
| + | |||
| + | (9) be created through an open process, in which all interested parties can be involved. | ||
| + | |||
| + | == '''Agenda''' == | ||
| + | |||
| + | '''<big><u>Wednesday, May 16 - Building 45 (Natcher), Balcony A</u></big>''' | ||
| + | |||
| + | * 8:00am -- Registration & Continental Breakfast | ||
| + | |||
| + | '''Session 1 (Chair: Barry Smith)''' | ||
| + | |||
| + | * 9:00am -- Carol Bean: ''Introduction'' | ||
| + | |||
| + | * 9:10am -- Susan Shurin, Deputy Director of the National Heart Lung and Blood Institute: ''Welcome'' | ||
| + | |||
| + | * 9:20am -- Werner Ceusters: ''How to Build an Ontology'' [http://ontology.buffalo.edu/07/CTO/ceusters.ppt ppt] | ||
| + | |||
| + | * 10:00am -- Discussion | ||
| + | |||
| + | * 10:15am -- Refreshment Break | ||
| + | |||
| + | '''Session 2 (Chair: Dave Parrish)''' | ||
| + | |||
| + | * 10:30am -- Chris Mungall: ''The OBO Foundry'' [http://ontology.buffalo.edu/07/CTO/mungall.ppt ppt] | ||
| + | |||
| + | * 11:00am -- Jennifer Fostel: ''The Ontology for Biomedical Investigations'' [http://ontology.buffalo.edu/07/CTO/fostel.ppt ppt] | ||
| + | |||
| + | * 11:30am -- Richard Scheuermann: ''Introducing the Clinical Trial Ontology'' [http://ontology.buffalo.edu/07/CTO/scheuermann.ppt ppt] | ||
| + | |||
| + | * 12:00pm -- Discussion | ||
| + | |||
| + | * 12:30pm -- Lunch Break | ||
| + | |||
| + | '''Session 3 (Chair: Werner Ceusters)''' | ||
| + | |||
| + | * 1:30pm -- Ida Sim: ''The RCT Schema'' [http://ontology.buffalo.edu/07/CTO/sim.ppt ppt] | ||
| + | |||
| + | * 2:00pm -- Amar Das: ''Epoch: An Ontological Framework for Clinical Trial Management'' | ||
| + | [http://ontology.buffalo.edu/07/CTO/Das.pdf ppt] | ||
| + | |||
| + | * 2:30pm -- Barry Smith: ''The Clinical Trial Ontology: Creating Consensus'' [http://ontology.buffalo.edu/07/CTO/smith.ppt ppt] | ||
| + | |||
| + | * 3:30pm -- Refreshment Break | ||
| + | |||
| + | * 4:00pm -- Discussion Session | ||
| + | |||
| + | * 5:00pm -- Close | ||
| + | |||
| + | |||
| + | '''<big><u>Thursday, May 17 - Building 31, Conference Room 10</u></big>''' | ||
| + | |||
| + | * 8:00am -- Registration & Continental Breakfast | ||
| + | |||
| + | '''Session 4 (Moderator: Jennifer Fostel)''' | ||
| + | |||
| + | * 9:00am -- Building the Clinical Trial Ontology | ||
| + | |||
| + | * 10:30am -- Refreshment Break | ||
| + | |||
| + | ''' Session 5 (Moderator: Alan Ruttenberg)''' | ||
| + | |||
| + | * 11:00am -- Applying the Clinical Trial Ontology | ||
| + | **Norbert Graf: ACGT's Clinical Trial Builder [http://ontology.buffalo.edu/07/CTO/graf.ppt ppt] | ||
| + | |||
| + | * 12:30pm -- Lunch Break | ||
| + | |||
| + | '''Session 6 (Moderator: Richard Scheuermann)''' | ||
| + | |||
| + | * 1:30pm -- The Future of the Clinical Trial Ontology [[Media:CTO_Workshop_Day_2_RS_JL.doc]] | ||
| + | |||
| + | * 3:00pm -- Close | ||
| + | |||
| + | == '''NIH Security Regulations''' == | ||
| + | |||
| + | All visitor vehicles, including taxicabs, hotel and airport shuttles, delivery trucks and vans will be inspected before being allowed on campus. Visitors will be asked to show a form of government-issued photo identification (driver's license, passport, green card, etc.) and to state the purpose of their visit. Be sure to allow extra time for this vehicle inspection procedure. Employees and visitors should continue to wear their identification prominently at all times while on campus. | ||
| + | |||
| + | Guards will remain at certain buildings to address specific program requirements such as sensitive research and safety concerns. At building entrances where guards are posted: | ||
| + | * Visitors may be required to log in, wear a visitors pass and have an employee escort them through the building. | ||
| + | * Visitors may be required to pass through a metal detector and have bags, backpacks or purses inspected or x-rayed as they enter buildings. | ||
| + | Security staff will be looking for and confiscating any suspicious or potentially dangerous materials. U.S. Code prohibits bringing any dangerous weapons onto Federal property, including anything with a blade longer than 2½ inches. Meeting participants may want to leave extra bags or personal materials at their hotel to minimize the time needed for inspection. | ||
| + | |||
| + | All visitors including patients, contractors, vendors and delivery persons must use the following entrances: | ||
| + | |||
| + | <u>Rockville Pike at South Drive</u> (Metro): | ||
| + | * Open 24 hours - Inbound and Outbound traffic | ||
| + | * Open to All Vehicles | ||
| + | * Pedestrian Turnstiles Open for Employees | ||
| + | * Gateway Center at Metro Open for Visitor Registration--this is where visitors check-in with security if they are not driving | ||
| + | |||
| + | <u>Old Georgetown Road at Center Drive</u> (This entrance is primarily for commercial vehicles and visitors): | ||
| + | * 6am- 7pm -Inbound and Outbound Traffic | ||
| + | * Open to Employee, Commercial and Visitor Vehicles | ||
| + | * Pedestrian Turnstiles Open for Employees | ||
| + | |||
| + | Please note: Visitor parking is limited at NIH. Visitors are encouraged to use public transportation such as the Metrorail subway system which has a convenient stop (Medical Center) on the NIH campus. | ||
| + | |||
| + | Visitors arriving by bus will be dropped off at the NIH/Medical Center Metro stop at Rockville Pike and South Drive. Patients and visitors on official business can then ride the Campus Shuttle to the Clinical Center and other designated shuttle stops on the campus. | ||
| + | |||
| + | == '''Participants (Final)''' == | ||
| + | |||
| + | Robert Arp -- NCBO, University at Buffalo | ||
| + | |||
| + | Elaine Ayres -- NIH / Clinical Center | ||
| + | |||
| + | William Barrick -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH | ||
| + | |||
| + | Carol Bean -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | Maureen Beanan -- National Center for Research Resources (NCRR) / NIH | ||
| + | |||
| + | Elmer Bernstam -- University of Texas Health Science Center at Houston | ||
| + | |||
| + | Olivier Bodenreider -- National Library of Medicine (NLM) / NIH | ||
| + | |||
| + | Olga Brazhnik -- National Center for Research Resources (NCRR) / NIH | ||
| + | |||
| + | Constantino Castillo -- The KEVRIC Company, Inc. | ||
| + | |||
| + | Werner Ceusters -- Ontology Research Group, University at Buffalo | ||
| + | |||
| + | Huey Cheung -- Center for Information Technology (CIT) / NIH | ||
| + | |||
| + | Ling Chin -- National Institute on Deafness and Other Communication Disorders (NIDCD) / NIH | ||
| + | |||
| + | In Hye Cho -- National Library of Medicine (NLM) / NIH | ||
| + | |||
| + | Christopher G. Chute -- NCBO, Mayo Clinic | ||
| + | |||
| + | Kevin Clany -- Invitrogen Corp. | ||
| + | |||
| + | Christian Cocos -- IFOMIS (Saarbrücken, Germany) | ||
| + | |||
| + | Elaine Collier -- National Center for Research Resources (NCRR) / NIH | ||
| + | |||
| + | Leo Cousineau -- Information Management Consultants (Reston, VA) | ||
| + | |||
| + | Lindsay Cowell -- Duke University Medical Center | ||
| + | |||
| + | Amar Das -- Stanford Medical Informatics | ||
| + | |||
| + | Kaushal Desai -- AstraZeneca | ||
| + | |||
| + | Stephen Dobson -- Pfizer Global Research and Development | ||
| + | |||
| + | Liju Fan -- Ontology Workshop, LLC | ||
| + | |||
| + | Gabriele Feolo -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | Kerstin Forsberg -- AstraZeneca | ||
| + | |||
| + | Jennifer Fostel -- NIH/NIEHS | ||
| + | |||
| + | Douglas Fridsma -- University of Pittsburgh | ||
| + | |||
| + | Jane Fun -- Contractor, National Library of Medicine (NLM) / NIH | ||
| + | |||
| + | Louis J. Goldberg -- University at Buffalo | ||
| + | |||
| + | Peter Good -- National Human Genome Research Institute (NHGRI) / NIH | ||
| + | |||
| + | Norbert Graf -- Clinic for Paediatric Oncology and Haematology, Saarland University (Homburg, Germany) | ||
| + | |||
| + | Lakshmi M. Grama -- National Cancer Institute (NCI) / NIH | ||
| + | |||
| + | Ted Grasela -- Cognigen Corporation (Amherst, NY) | ||
| + | |||
| + | Herb Hagler -- University of Texas Southwestern Medical Center | ||
| + | |||
| + | Andrea Harabin -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | William Harlan -- Consultant, National Library of Medicine (NLM) / NIH | ||
| + | |||
| + | Steve Harris -- Computing Laboratory, University of Oxford | ||
| + | |||
| + | Frank Hartel -- NCI Center for Bioinformatics / NIH | ||
| + | |||
| + | Calvin A. Johnson -- Center for Information Technology (CIT) / NIH | ||
| + | |||
| + | Heather A. Junkins -- CRIIT & NHLBI / NIH | ||
| + | |||
| + | Webster Kelsey -- Dept of Biomedical Informatics, University of Pittsburgh | ||
| + | |||
| + | Warren A. Kibbe -- Northwestern University | ||
| + | |||
| + | Bron W. Kisler -- Clinical Data Interchange Standards Consortium (CDISC) | ||
| + | |||
| + | George A. Komatsoulis -- NCI Center for Bioinformatics / NIH | ||
| + | |||
| + | Jennie Larkin -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | William Lau -- Center for Information Technology (CIT) / NIH | ||
| + | |||
| + | Jamie Lee -- University of Texas Southwestern Medical Center at Dallas | ||
| + | |||
| + | Natasha Levenkov -- Rockefeller University | ||
| + | |||
| + | Yuan Liu -- National Institute of Neurological Diseases and Stroke (NINDS) / NIH | ||
| + | |||
| + | Yun Lu -- KAI Research, Inc. | ||
| + | |||
| + | Dan Lyman -- Information Management Consultants (Reston, VA) | ||
| + | |||
| + | Peter Lyster -- Center for Bioinformatics and Computational Biology, National Institute of General Medical Sciences (NIGMS) / NIH | ||
| + | |||
| + | Peter Maccallum -- CRUK Cambridge Research Institute, University of Cambridge | ||
| + | |||
| + | Charles Mead -- NCI/NIH, Center for Biomedical Informatics and Information Technology | ||
| + | |||
| + | Chris Mungall -- NCBO, Lawrence Berkeley National Laboratory | ||
| + | |||
| + | Robert A. Musson -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | Darren Natale -- Georgetown University Medical Center | ||
| + | |||
| + | Fabian Neuhaus -– National Institute of Standards and Technology (NIST) | ||
| + | |||
| + | Eric Neumann -- Teranode (Seattle, WA) | ||
| + | |||
| + | Eduardo Ortiz -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | Jeng-Jong Pan -- National Institute on Drug Abuse (NIDA) / NIH | ||
| + | |||
| + | Dave Parrish -- Immune Tolerance Network (Pittsburgh, PA) | ||
| + | |||
| + | George O. Redmond -- National Cancer Institute (NCI) / NIH | ||
| + | |||
| + | Dianne M. Reeves -- National Cancer Institute, Center for Biomedical Informatics and Information Technology (formerly NCICB) / NIH | ||
| + | |||
| + | Alan Ruttenberg -- Science Commons (c/o MIT CSAIL) | ||
| + | |||
| + | Jody Sachs -- National Center for Research Resources (NCRR) / NIH | ||
| + | |||
| + | Michael Sayre -- National Center for Research Resources (NCRR) / NIH | ||
| + | |||
| + | Richard Scheuermann -- University of Texas Southwestern Medical Center | ||
| + | |||
| + | Susan Shurin -- National Heart, Lung and Blood Institute (NHLBI) / NIH | ||
| + | |||
| + | Hua-Chuan Sim -- National Library of Medicine (NLM) / NIH | ||
| + | |||
| + | Ida Sim -- Trial Bank, University of California at San Francisco Medical Center | ||
| − | + | Barry Smith -- NCBO, University at Buffalo | |
| − | + | Ranjana Srivastava -- Information Management Consultants (Reston, VA) | |
| − | |||
| − | |||
| + | Samson Tu -- Stanford University | ||
| − | + | Suresh Varghese -- Digital Infuzion, Inc. | |
| − | + | Chunhua Weng -- University of Pittsburgh | |
| + | Rebecca Williams -- National Library of Medicine (NLM) / NIH | ||
| − | + | Ashley Xia -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH | |
| − | + | Sandhya Xirasagar -- Information Management Consultants (Reston, VA) | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | Alison Yao -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH | ||
| − | + | Zhensheng Zhang -- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) / NIH | |
| + | == '''Venue''' == | ||
| − | + | Day 1: Balcony A, Building 45 (Natcher), NIH Campus, Bethesda, MD | |
| + | Day 2: Conference Room 10, Building 31, NIH Campus, Bethesda, MD | ||
| − | + | A printable NIH Visitors Map can be found at: [http://parking.nih.gov/documents/visitorAccessMap.pdf NIH Visitors Map] | |
| − | |||
| + | A printable schedule of shuttle service between area hotels and NIH can be found at: [http://dtts.ors.od.nih.gov/documents/south_hotel_shuttles.pdf NIH Shuttles] | ||
| − | + | == '''Links''' == | |
| + | [http://www.bioontology.org/ncbo/faces/index.xhtml NCBO Bioportal] | ||
| − | + | [http://obo.sourceforge.net Open Biomedical Ontologies] | |
| − | + | [http://obofoundry.org OBO Foundry] | |
| − | + | [http://obi.sourceforge.net Ontology for Biomedical Investigations] | |
| − | |||
| − | + | [http://rctbank.ucsf.edu/ Trial Bank] | |
| − | + | [http://protege.stanford.edu/conference/2006/submissions/slides/3.2_Shankar_EpochModels.pdf Epoch Ontology] | |
| − | + | [http://www.ifomis.uni-saarland.de/wiki/index.php/ACGT The ACGT Project]: Advancing Clinico-Genomic Trials on Cancer | |
| − | + | [http://www.bioontology.org/wiki/index.php/CTO:Main_Page Clinical Trial Ontology Wiki] | |
| − | + | [http://esw.w3.org/topic/HCLS/OntologyTaskForce/BIONTDSEDCM HCLS Ontology Task Force] | |
| − | + | A video introduction to ontologies by Barry Smith is available here: | |
| − | + | *Realmedia: rtsp://stream.buffalo.edu/shared/research/phismith/Stanford10-25-06/Tutorial.rm | |
| − | + | *Mediaplayer: rtsp://stream.buffalo.edu/shared/research/phismith/Stanford10-25-06/Tutorial.WMV | |
| − | + | The second half of this presentation pertains to the building of the Clinical Trial Ontology. | |
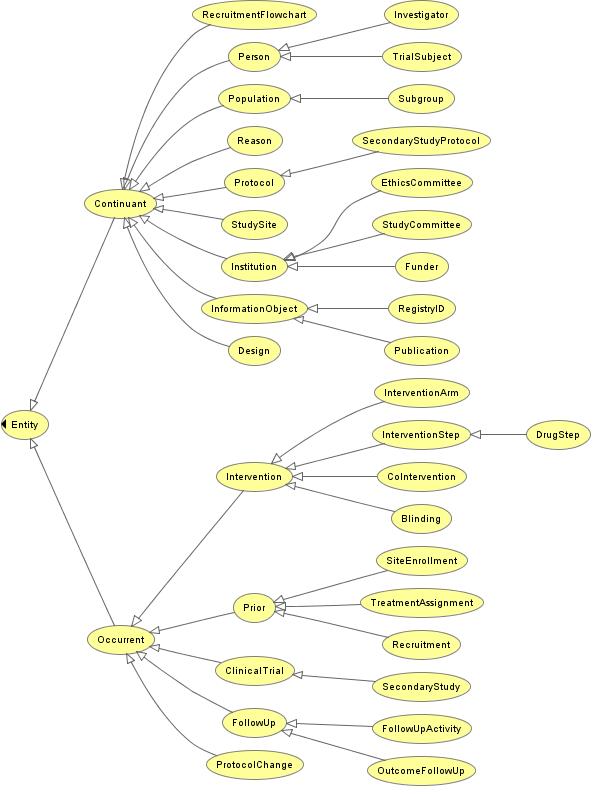
| − | + | == '''Preliminary Fragment of CTO''' (for illustration purposes only) == | |
| − | + | [http://cto.googlecode.com/svn/trunk/CTO.owl Current OWL version] | |
| − | + | [[Image:CTO.jpg]] | |
Latest revision as of 11:38, 6 August 2008
General Information
The National Center for Biomedical Ontology will host a two-day workshop focused on the Ontology of Clinical Trials. The workshop will take place on May 16-17, 2007 at the NIH Campus in Bethesda, MD.
The meeting will be divided into two parts. Day 1 will be a public event consisting of presentations and panel discussions on the CTO and cognate initiatives. Day 2 will be a working meeting devoted to intense discussions of mature drafts of the CTO and to the creation of a strategy for its further development and testing.
The work of the NCBO is funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant 1 U 54 HG004028. Information on the National Centers for Biomedical Computing can be found here.
We are grateful to the National Heart, Lung and Blood Institute for generous support for this meeting.
Aim of the Workshop
The aim of the workshop is to foster the creation and dissemination of a reference ontology (high-quality controlled structured vocabulary) for the annotation of the results of clinical trials.
The Workshop will bring together representatives of all of the major groups involved in clinical trial informatics, design, execution, analysis and standardization, with the goal of achieving a broad consensus on the requirements which the ontology should address and on the most effective means of realizing these requirements. It will address the potential uses of CTO, and methodology for CTO design and development.
Goals of the Clinical Trial Ontology Initiative
The proposed CTO should:
(1) fully and faithfully capture the types of entities and relationships involved in clinical trials of any experimental design
(2) comprehend terms like: cohort, randomization, placebo, response, efficacy, control, protocol, null hypothesis, confidence interval, finding, biomarker, primary outcome, secondary outcome, intervention group, confounder, etc., including also major relevant statistical terms and terms drawn from resources such as the CDISC glossary and all terms needed for the task of meta-analysis of clinical trials;
(3) organize these terms in a structured way, providing definitions and logical relations designed to enhance retrieval of, reasoning with, and integration of the data annotated in its terms.
(4) support trial bank interoperation
(5) form an integral part of the more comprehensive framework of the Ontology for Biomedical Investigations OBI, which itself forms part of the OBO Foundry
(6) draw on and seek maximal alignment with existing clinical trial ontologies, including:
-- the Epoch ontology used by the Immune Tolerance Network
-- the RCT Schema ontology used by theTrial Bank Initiative
(7) rest on a clear understanding of the relation between CTO and related ventures such as an epidemiology study ontology
(8) rest on a clear understanding of the relation between CTO and data-model-oriented initiatives such as CDISC and BRIDG
(9) be created through an open process, in which all interested parties can be involved.
Agenda
Wednesday, May 16 - Building 45 (Natcher), Balcony A
- 8:00am -- Registration & Continental Breakfast
Session 1 (Chair: Barry Smith)
- 9:00am -- Carol Bean: Introduction
- 9:10am -- Susan Shurin, Deputy Director of the National Heart Lung and Blood Institute: Welcome
- 9:20am -- Werner Ceusters: How to Build an Ontology ppt
- 10:00am -- Discussion
- 10:15am -- Refreshment Break
Session 2 (Chair: Dave Parrish)
- 10:30am -- Chris Mungall: The OBO Foundry ppt
- 11:00am -- Jennifer Fostel: The Ontology for Biomedical Investigations ppt
- 11:30am -- Richard Scheuermann: Introducing the Clinical Trial Ontology ppt
- 12:00pm -- Discussion
- 12:30pm -- Lunch Break
Session 3 (Chair: Werner Ceusters)
- 1:30pm -- Ida Sim: The RCT Schema ppt
- 2:00pm -- Amar Das: Epoch: An Ontological Framework for Clinical Trial Management
- 2:30pm -- Barry Smith: The Clinical Trial Ontology: Creating Consensus ppt
- 3:30pm -- Refreshment Break
- 4:00pm -- Discussion Session
- 5:00pm -- Close
Thursday, May 17 - Building 31, Conference Room 10
- 8:00am -- Registration & Continental Breakfast
Session 4 (Moderator: Jennifer Fostel)
- 9:00am -- Building the Clinical Trial Ontology
- 10:30am -- Refreshment Break
Session 5 (Moderator: Alan Ruttenberg)
- 11:00am -- Applying the Clinical Trial Ontology
- Norbert Graf: ACGT's Clinical Trial Builder ppt
- 12:30pm -- Lunch Break
Session 6 (Moderator: Richard Scheuermann)
- 1:30pm -- The Future of the Clinical Trial Ontology Media:CTO_Workshop_Day_2_RS_JL.doc
- 3:00pm -- Close
NIH Security Regulations
All visitor vehicles, including taxicabs, hotel and airport shuttles, delivery trucks and vans will be inspected before being allowed on campus. Visitors will be asked to show a form of government-issued photo identification (driver's license, passport, green card, etc.) and to state the purpose of their visit. Be sure to allow extra time for this vehicle inspection procedure. Employees and visitors should continue to wear their identification prominently at all times while on campus.
Guards will remain at certain buildings to address specific program requirements such as sensitive research and safety concerns. At building entrances where guards are posted:
- Visitors may be required to log in, wear a visitors pass and have an employee escort them through the building.
- Visitors may be required to pass through a metal detector and have bags, backpacks or purses inspected or x-rayed as they enter buildings.
Security staff will be looking for and confiscating any suspicious or potentially dangerous materials. U.S. Code prohibits bringing any dangerous weapons onto Federal property, including anything with a blade longer than 2½ inches. Meeting participants may want to leave extra bags or personal materials at their hotel to minimize the time needed for inspection.
All visitors including patients, contractors, vendors and delivery persons must use the following entrances:
Rockville Pike at South Drive (Metro):
- Open 24 hours - Inbound and Outbound traffic
- Open to All Vehicles
- Pedestrian Turnstiles Open for Employees
- Gateway Center at Metro Open for Visitor Registration--this is where visitors check-in with security if they are not driving
Old Georgetown Road at Center Drive (This entrance is primarily for commercial vehicles and visitors):
- 6am- 7pm -Inbound and Outbound Traffic
- Open to Employee, Commercial and Visitor Vehicles
- Pedestrian Turnstiles Open for Employees
Please note: Visitor parking is limited at NIH. Visitors are encouraged to use public transportation such as the Metrorail subway system which has a convenient stop (Medical Center) on the NIH campus.
Visitors arriving by bus will be dropped off at the NIH/Medical Center Metro stop at Rockville Pike and South Drive. Patients and visitors on official business can then ride the Campus Shuttle to the Clinical Center and other designated shuttle stops on the campus.
Participants (Final)
Robert Arp -- NCBO, University at Buffalo
Elaine Ayres -- NIH / Clinical Center
William Barrick -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH
Carol Bean -- National Heart, Lung and Blood Institute (NHLBI) / NIH
Maureen Beanan -- National Center for Research Resources (NCRR) / NIH
Elmer Bernstam -- University of Texas Health Science Center at Houston
Olivier Bodenreider -- National Library of Medicine (NLM) / NIH
Olga Brazhnik -- National Center for Research Resources (NCRR) / NIH
Constantino Castillo -- The KEVRIC Company, Inc.
Werner Ceusters -- Ontology Research Group, University at Buffalo
Huey Cheung -- Center for Information Technology (CIT) / NIH
Ling Chin -- National Institute on Deafness and Other Communication Disorders (NIDCD) / NIH
In Hye Cho -- National Library of Medicine (NLM) / NIH
Christopher G. Chute -- NCBO, Mayo Clinic
Kevin Clany -- Invitrogen Corp.
Christian Cocos -- IFOMIS (Saarbrücken, Germany)
Elaine Collier -- National Center for Research Resources (NCRR) / NIH
Leo Cousineau -- Information Management Consultants (Reston, VA)
Lindsay Cowell -- Duke University Medical Center
Amar Das -- Stanford Medical Informatics
Kaushal Desai -- AstraZeneca
Stephen Dobson -- Pfizer Global Research and Development
Liju Fan -- Ontology Workshop, LLC
Gabriele Feolo -- National Heart, Lung and Blood Institute (NHLBI) / NIH
Kerstin Forsberg -- AstraZeneca
Jennifer Fostel -- NIH/NIEHS
Douglas Fridsma -- University of Pittsburgh
Jane Fun -- Contractor, National Library of Medicine (NLM) / NIH
Louis J. Goldberg -- University at Buffalo
Peter Good -- National Human Genome Research Institute (NHGRI) / NIH
Norbert Graf -- Clinic for Paediatric Oncology and Haematology, Saarland University (Homburg, Germany)
Lakshmi M. Grama -- National Cancer Institute (NCI) / NIH
Ted Grasela -- Cognigen Corporation (Amherst, NY)
Herb Hagler -- University of Texas Southwestern Medical Center
Andrea Harabin -- National Heart, Lung and Blood Institute (NHLBI) / NIH
William Harlan -- Consultant, National Library of Medicine (NLM) / NIH
Steve Harris -- Computing Laboratory, University of Oxford
Frank Hartel -- NCI Center for Bioinformatics / NIH
Calvin A. Johnson -- Center for Information Technology (CIT) / NIH
Heather A. Junkins -- CRIIT & NHLBI / NIH
Webster Kelsey -- Dept of Biomedical Informatics, University of Pittsburgh
Warren A. Kibbe -- Northwestern University
Bron W. Kisler -- Clinical Data Interchange Standards Consortium (CDISC)
George A. Komatsoulis -- NCI Center for Bioinformatics / NIH
Jennie Larkin -- National Heart, Lung and Blood Institute (NHLBI) / NIH
William Lau -- Center for Information Technology (CIT) / NIH
Jamie Lee -- University of Texas Southwestern Medical Center at Dallas
Natasha Levenkov -- Rockefeller University
Yuan Liu -- National Institute of Neurological Diseases and Stroke (NINDS) / NIH
Yun Lu -- KAI Research, Inc.
Dan Lyman -- Information Management Consultants (Reston, VA)
Peter Lyster -- Center for Bioinformatics and Computational Biology, National Institute of General Medical Sciences (NIGMS) / NIH
Peter Maccallum -- CRUK Cambridge Research Institute, University of Cambridge
Charles Mead -- NCI/NIH, Center for Biomedical Informatics and Information Technology
Chris Mungall -- NCBO, Lawrence Berkeley National Laboratory
Robert A. Musson -- National Heart, Lung and Blood Institute (NHLBI) / NIH
Darren Natale -- Georgetown University Medical Center
Fabian Neuhaus -– National Institute of Standards and Technology (NIST)
Eric Neumann -- Teranode (Seattle, WA)
Eduardo Ortiz -- National Heart, Lung and Blood Institute (NHLBI) / NIH
Jeng-Jong Pan -- National Institute on Drug Abuse (NIDA) / NIH
Dave Parrish -- Immune Tolerance Network (Pittsburgh, PA)
George O. Redmond -- National Cancer Institute (NCI) / NIH
Dianne M. Reeves -- National Cancer Institute, Center for Biomedical Informatics and Information Technology (formerly NCICB) / NIH
Alan Ruttenberg -- Science Commons (c/o MIT CSAIL)
Jody Sachs -- National Center for Research Resources (NCRR) / NIH
Michael Sayre -- National Center for Research Resources (NCRR) / NIH
Richard Scheuermann -- University of Texas Southwestern Medical Center
Susan Shurin -- National Heart, Lung and Blood Institute (NHLBI) / NIH
Hua-Chuan Sim -- National Library of Medicine (NLM) / NIH
Ida Sim -- Trial Bank, University of California at San Francisco Medical Center
Barry Smith -- NCBO, University at Buffalo
Ranjana Srivastava -- Information Management Consultants (Reston, VA)
Samson Tu -- Stanford University
Suresh Varghese -- Digital Infuzion, Inc.
Chunhua Weng -- University of Pittsburgh
Rebecca Williams -- National Library of Medicine (NLM) / NIH
Ashley Xia -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH
Sandhya Xirasagar -- Information Management Consultants (Reston, VA)
Alison Yao -- National Institute of Allergy and Infectious Diseases (NIAID) / NIH
Zhensheng Zhang -- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) / NIH
Venue
Day 1: Balcony A, Building 45 (Natcher), NIH Campus, Bethesda, MD
Day 2: Conference Room 10, Building 31, NIH Campus, Bethesda, MD
A printable NIH Visitors Map can be found at: NIH Visitors Map
A printable schedule of shuttle service between area hotels and NIH can be found at: NIH Shuttles
Links
Ontology for Biomedical Investigations
The ACGT Project: Advancing Clinico-Genomic Trials on Cancer
A video introduction to ontologies by Barry Smith is available here:
- Realmedia: rtsp://stream.buffalo.edu/shared/research/phismith/Stanford10-25-06/Tutorial.rm
- Mediaplayer: rtsp://stream.buffalo.edu/shared/research/phismith/Stanford10-25-06/Tutorial.WMV
The second half of this presentation pertains to the building of the Clinical Trial Ontology.